Complicated intra-abdominal infections
Adult: Available preparation:
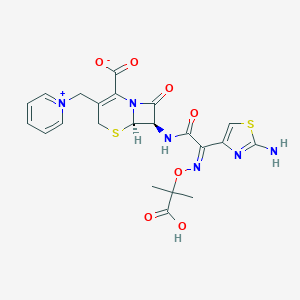
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
In combination with metronidazole, and with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 5-14 days.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
In combination with metronidazole, and with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 5-14 days. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
In combination with metronidazole, and with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 5-14 days.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
In combination with metronidazole, and with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 5-14 days. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Intravenous
Complicated urinary tract infections
Adult: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including pyelonephritis: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 5-10 days, may be increased to 14 days in patients with bacteraemia. Total duration of treatment may include IV doses, followed by appropriate oral therapy.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including pyelonephritis: In combination with antibacterial drug active against Gram-positive pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 5-14 days. Total duration of treatment may include IV doses, followed by appropriate oral therapy. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including pyelonephritis: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 5-10 days, may be increased to 14 days in patients with bacteraemia. Total duration of treatment may include IV doses, followed by appropriate oral therapy.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including pyelonephritis: In combination with antibacterial drug active against Gram-positive pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 5-14 days. Total duration of treatment may include IV doses, followed by appropriate oral therapy. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Intravenous
Hospital-acquired pneumonia
Adult: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including ventilator-associated pneumonia: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 7-14 days.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including ventilator-associated pneumonia: In combination with antibacterial drug(s) active against Gram-positive pathogens and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 7-14 days. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including ventilator-associated pneumonia: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours for 7-14 days.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases including ventilator-associated pneumonia: In combination with antibacterial drug(s) active against Gram-positive pathogens and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration: 7-14 days. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Intravenous
Bacteraemia
Adult: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases associated with or suspected to be associated with complicated intra-abdominal infections, complicated UTI, or hospital-acquired pneumonia (HAP): 2 g/0.5 g 8 hourly via infusion over 2 hours. Treatment duration may vary according to the site of infection.
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases associated with or suspected to be associated with complicated intra-abdominal infections, complicated UTI, or hospital-acquired pneumonia (HAP): 2 g/0.5 g 8 hourly via infusion over 2 hours. Treatment duration may vary according to the site of infection.
Intravenous
Susceptible Gram-negative infections
Adult: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases due to aerobic organisms in patients with limited treatment options: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours. Treatment duration may vary depending on the severity of the infection, pathogen(s), and clinical response of the patient.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases due to aerobic organisms in patients with limited treatment options: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration may vary depending on the severity of the infection, pathogen(s), and clinical and bacteriological progress of the patient. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases due to aerobic organisms in patients with limited treatment options: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 2 g/0.5 g 8 hourly via infusion over 2 hours. Treatment duration may vary depending on the severity of the infection, pathogen(s), and clinical response of the patient.
Child: Available preparation:
Ceftazidime 2 g and avibactam 0.5 g powder for solution for infusion
For cases due to aerobic organisms in patients with limited treatment options: In combination with antibacterial drug(s) active against Gram-positive and/or anaerobic pathogens (when these are known or suspected to contribute to the infection): 3-<6 months 0.04 g/0.01 g/kg 8 hourly; 6 months to <18 years 0.05 g/0.0125 g/kg 8 hourly, up to Max of 2 g/0.5 g. All doses are given via infusion for over 2 hours. Treatment duration may vary depending on the severity of the infection, pathogen(s), and clinical and bacteriological progress of the patient. Treatment recommendations may vary among countries and individual products (refer to local detailed product guideline).




 Đăng xuất
Đăng xuất